롱
$SIOX PT 10-19 and higher
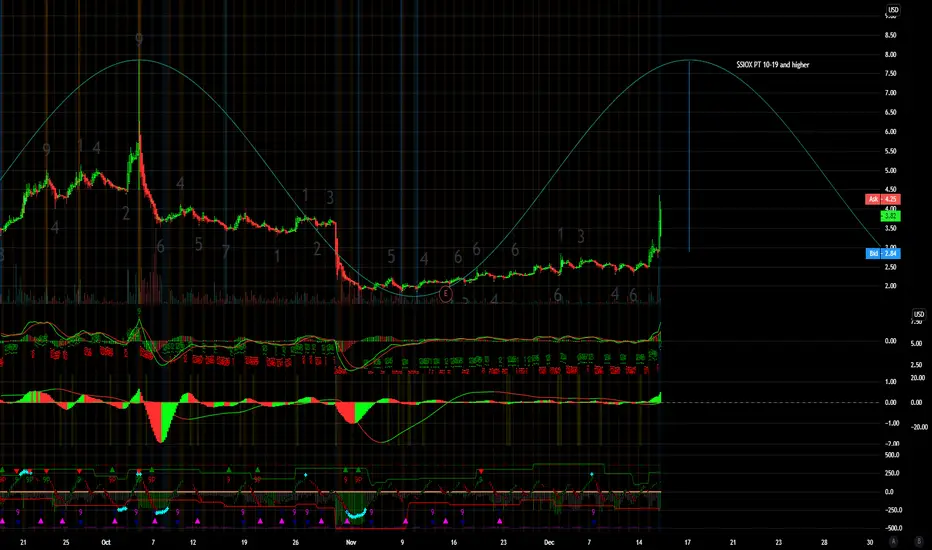
Sio Gene Therapies Announces Positive Six-Month Follow-Up Data from Low-Dose Cohort of Phase 1/2 Trial of AXO-AAV-GM1 for GM1 Gangliosidosis
- Generally well-tolerated with a favorable safety profile in five patients
- Serum beta-galactosidase enzyme activity increased in all patients at all timepoints between Day 7 and Month 6, representing an approximate doubling in enzyme activity after gene transfer
- At Month 6, enzyme activity restored to 23-57% (mean: 38%) of normal reference levels
- All five children demonstrated signs of clinical disease stability as assessed by Vineland-3 Growth Scale Value, Upright and Floor Mobility, and Clinical Global Impression (CGI) scales
- High-dose cohort initiated in November 2020; two patients now dosed without complications
- Generally well-tolerated with a favorable safety profile in five patients
- Serum beta-galactosidase enzyme activity increased in all patients at all timepoints between Day 7 and Month 6, representing an approximate doubling in enzyme activity after gene transfer
- At Month 6, enzyme activity restored to 23-57% (mean: 38%) of normal reference levels
- All five children demonstrated signs of clinical disease stability as assessed by Vineland-3 Growth Scale Value, Upright and Floor Mobility, and Clinical Global Impression (CGI) scales
- High-dose cohort initiated in November 2020; two patients now dosed without complications
면책사항
해당 정보와 게시물은 금융, 투자, 트레이딩 또는 기타 유형의 조언이나 권장 사항으로 간주되지 않으며, 트레이딩뷰에서 제공하거나 보증하는 것이 아닙니다. 자세한 내용은 이용 약관을 참조하세요.
면책사항
해당 정보와 게시물은 금융, 투자, 트레이딩 또는 기타 유형의 조언이나 권장 사항으로 간주되지 않으며, 트레이딩뷰에서 제공하거나 보증하는 것이 아닙니다. 자세한 내용은 이용 약관을 참조하세요.